Ijraset Journal For Research in Applied Science and Engineering Technology
- Home / Ijraset
- On This Page
- Abstract
- Introduction
- Conclusion
- References
- Copyright
Formulation Development and Cevaluation of Mucoadhesive Patch for Diabetes Using Plant Based Polysaccharide
Authors: Tapade S. S, Priyanka P. Dappadwad, Priti M. Mane, Punam P. Suryawanshi, Rameshwar Sangle, Ramraje Lahade, Rutuja Tandle, Rutuja Bedre
DOI Link: https://doi.org/10.22214/ijraset.2023.56312
Certificate: View Certificate
Abstract
Using plant-based polysaccharides, this study created a mucoadhesive patch for diabetes with the goal of delivering regulated medication release and improved therapeutic efficacy through sustained contact with the buccal mucosa. The patches\' physicochemical characteristics, drug release kinetics, and mucoadhesive strength were examined after they had been made using the solvent casting procedure. . The created patch had exceptional mechanical, flexible, and physical qualities, as well as prolonged drug release and minimized burst release. Through in vitro cytotoxicity tests, the patch\'s biocompatibility was verified, demonstrating its suitability for buccal administration.the buccal region offcers and attractive route of administration for systemic drug delivery. The patches were prepared by solvent casting method the design of patches were evaluated for thickness, uniformity ,folding ,endurance ,weight uniformity ,swelling behavior,determination of mucoadhesive time. . To confirm the effectiveness and safety of this unique medication delivery method, additional in vivo research and clinical trials are required. If successful, these studies could pave the way for more individualized and efficient diabetic treatment options.
Introduction
I. INTRODUCTION
To proficiently control helpful proteins/peptides, there have been a few endeavors to make inventive oral conveyance frameworks.The utilization of nanoparticles for insulin conveyance has gotten a ton of consideration as of late. For the oral organization of helpful proteins like salmon calcitonin, exenatide, and insulin, our gathering has been dealing with the advancement of mucoadhesive digestive gadgets. To arrive at the small digestive system, mucoadhesive gadgets are encased in intestinal covered containers created from a mix of mucoadhesive polymers. At the point when ingested, the containers fall to pieces in the stomach, delivering the gadgets, which then append to the digestive mucosa, extend, and step by step discharge their pharmacological burden as the gadget lattice breaks down. The gadgets remember a waterimpermeable covering for all sides with the exception of one, which takes into consideration controlled, one-way prescription delivery. Peptide and protein-based medicines are right now the focal point of medication advancement, making up close to half of the drug business' pipeline meds. This is on the grounds that these macromolecules can join just to their expected targets, diminishing the probability of undesirable aftereffects. Peptide/protein-based meds, then again, require parenterorganization due to their flimsiness in the GIT and restricted penetrability across natural filmsduring oral conveyance. Resistance with infusions presents a serious hindrance for habitually regulated meds like insulin, where inadequate control of diabetes can prompt difficult issue. As well as safeguarding the medicine from the stomach acidic climate, this gadgets block the proteolytic compound in the git from arriving the medication load. The gadget likewise produce area of strength for a focus slope,with support the retention of mediction conveying proteins across the GIT hindrance.
II. ADVANTAGES
- Mdds offers several advntages over other controlled oral controlled release system by
- virtue of prolongationresidenceof drug in Git.
- Targeting & localization of the dosage form at a specific site.
- High drug flux at the absorbing tissue
- Excellent accessibility
- Painless administration
- Low enzymatic activity & avoid of first pass metabolism
III. DISADVANAGES
- Relatively smaller area of absorption
- The thickness of delivery system should be limited to a few millimeter in order to avoid inconveniences for patient
- Part of drug may be dissolve in saliva and may be swallowed
- Drug which imitate oral mucosa or hve bitter taste cause allergy reaction
- If formulation contain antimicrobial agent ,affect the natural microbial flora
IV. EXPERIMENTAL SECTION
A. Materials
Evonik Ventures (Parsipanny, NJ, USA) was generous enough to donate polymers such as Eudragit® E PO and Eudragit® L100. Gelatin, carbopol, sodium carboxymethylcellulose (SCMC), polyvinylpyrrolidone (PPS) and ethylcellulose were purchased entirely from Sigma-Aldrich (St. Louis, MO, USA). Fisher Logical (Pittsburgh, Dad, USA) donated metoclopramide hydrochloride, streptozotocin (STZ), pH 4.5 sodium citrate buffer, and phosphate-buffered saline (PBS) tablets used inthisreview.
B. MUCOADHESION Study
Gupta et al. portrayed a trial in which pig digestive tract was utilized to examine the viability of mucoadhesion between clinical gadgets and the gastrointestinal mucosa. Pig gastrointestinal portions estimating 5 cm by 5 cm were refined in phosphate-buffered saline (PBS) at a pH of 7.4. The 5 mm gadgets had their support layers confronting away from the digestive surface and were shaken delicately at 37 degrees Celsius for 30 minutes.
C. MUCOADHESIVE Polymer
Mucoadhesive polymers include a large and diverse group of molecules covering biodegradable grafted co-polymers and thiolated polymers and are used in bioadhesive formulations either alone or in combination with others. These formulations are often watersoluble and when in dry form, they attract water from the biological surface and this water transfer results in a strong interaction [16]. The ideal mucoadhesive polymer should possess certain characteristics regarded as essential for effective function as a bioadhesive drug delivery system [19]. These include being non-toxic and non-irritant, possessing good spreading, swelling, solubility and biodegradable properties. In addition, they should possess adhesive properties both in the dry and liquid/gel state, be biocompatible and possess good viscoelastic, peel, tensile and shear strength properties as well as demonstrate local enzyme inhibition and penetration enhancement properties.
D. Polysaccharides
Polysaccharides are carbohydrates made up repeating monosaccharide or disaccharide units joined together by glycosidic bonds such as starch and glycogen. Most polysaccharides are naturally occurring which make them attractive choices in traditional applications as food and pharmaceutical additives or in the form of excipients as binders, sweeteners, bulking agents, film coatings and suspending agents. Further, polysaccharides are abundantly present in nature, have wide availability, are inexpensive and are available in a variety of structures with varied properties .
They can easily be modified chemically and biochemically and are highly stable, safe, nontoxic, hydrophilic and gel forming and in addition biodegradable. These include naturally occurring polysaccharides obtained from plant (e.g. guar gum, inulin), animal (e.g. chitosan and chondroitin sulphate), algae (e.g. alginates, xanthan) or microorganism (e.g. dextran) origin as well as starch and certain cellulosic polymers mainly from plant sources. The most common polysaccharides used as mucoadhesive polymer can be further divided into positively charged and negatively charged polysaccharide based on their charge property. The most commonly used positively charged polysaccharide is chitosan while the most commonly used negatively charged polysaccharides are alginate, pectin and hyaluronic acid.
E. MUCOADHESIVE Drug Delivery System
Drug delivery is most commonly achieved via oral administration of dosage forms such as tablets, capsules and liquids, representing about 70% of all pharmaceutical drug formulations. However, this is fraught with several problems including first pass metabolism in the liver, degradation in the gastrointestinal tract for acid labile drugs such as proteins and peptides and risk of poor uptake for children, the severely infirmed (e.g. comatose patients) and geriatric patients. The rejection rate of such oral dosage forms is higher than for other routes, due to factors such as unpleasant taste [1], difficulty in swallowing and the risk of choking. Though the alternative traditional parenteral route using injections is effective and avoids the above limitations, it presents several challenges as well, including pain, irritation at site of injection and the need for highly trained personnel for safe and effective administration. All these result in poor patient compliance with consequent poor clinical outcomes, which can be severe in certain diseases. There has been increased efforts in recent decades to develop novel alternative systems for drug delivery based on factors such as therapeutic concerns, biopharmaceutics and physico-chemical properties of the drug, such as poor solubility and instability via tradition routes
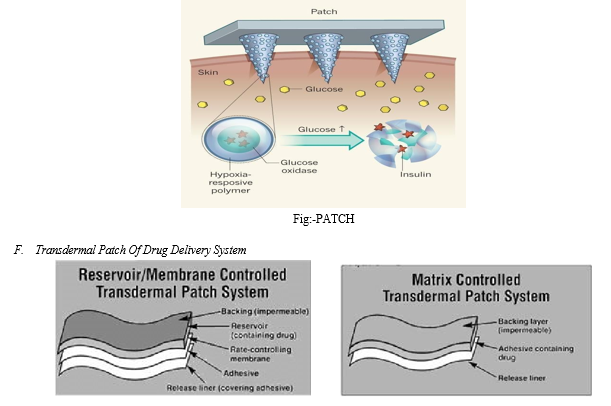
Transdermal drug delivery systems (TDDS), also known as ‘‘patches,’’ are dosage forms designed to deliver a therapeutically effective amount of drug across a patient’s skin. Several TDDS containing drugs such as clonidine, estradiol, fentanyl, nicotine, nitroglycerin, oxybutynin and scopoloamine are available in the United States.
In the Drug Quality Reporting System (DQRS), the United States Food and Drug Administration (FDA) has received numerous reports of ‘‘adhesion lacking’’ for transdermal drug delivery systems.
- Reservoir
Unlike the single-layer and multi-layer drug-in-adhesive systems, the reservoir transdermal system has a separate drug layer. The drug layer is a liquid compartment containing a drug solution or suspension separated by the adhesive layer. The drug reservoir is totally encapsulated in a shallow compartment molded from a drug-impermeable metallic plastic laminate, with a rate-controlling membrane made of a polymer like vinyl acetate on one surface. This patch is also backed by the backing layer. In this type of system the rate of release is zero order.
2. Matrix
The matrix system has a drug layer of a semisolid matrix containing a drug solution or suspension. The adhesive layer in this patch surrounds the drug layer, partially overlaying it. Also known as a monolithic device.
G. In Vivo Efficacy Studies
The creature tests were directed as per the principles set out by the College of California, St Nick Barbara's creature care board of trustees and the Organization of Research facility Creature Assets' Aide for the Consideration and Utilization of Creatures.
H. Studies Of Effectiveness In Rats Without Diabetes
Ordinary male Wistar rodents gauging somewhere in the range of 250 and 350 grams were utilized in the tests. Every one of the four gatherings of creatures comprised of six creatures, and the fifth gathering had just three creatures. The creatures were abstained for the earlier evening yet had unlimited admittance to water until the day of the preliminary. The day of the investigation, the rodents were given a subcutaneous infusion of 5 mg/kg metoclopramide hydrochloride to prompt gastricexhausting, and afterward they were given cases containing either void gadgets, insulin gadgets, insulin gadgets with remotely present PPS, or insulin gadgets with inner PPS.
I. Animal Model For Type 2 Diabetes
Diabetes was prompted in male Wistar rodents gauging somewhere in the range of 250 and 350 g. Short-term, the creatures were famished yet had full admittance to water. Utilizing a business glucose meter, we estimated the subjects' fasting glucose levels in the tail vein. From that point onward, 55 mg/kg STZ made in vivo was conveyed intraperitoneally into the mice.
J. Diabetic Rat Trials Of Efficacy
Following diabetes acceptance, the creatures were abstained for the time being nevertheless permitted free admittance to water, and afterward split into seven gatherings of six creatures each and one gathering of three. In the first six groups, Insulin Devices, Insulin Devices with 10% wt/wt PPS Instruments (0.6mg PPS/organism), Insulin Devices with 5mg PPS are remotely available in the box, empty and set up. insulinPPS (5mg) was definitely given orally.
K. Statistical Analyses
Data are shown as mean SE. Measurable significance was resolved using Understudy's one-way oscillation test or oneway volatility test (ANOVA) with appropriate post-hoc investigation (Graphpad, Crystal 6.0, GraphPad Programming, La Jolla, CA, USA).
V. RESULTS AND DISCUSSION
The contraptions were set up in the way recently expressed. Cases containing mucoadhesive patches were intestinally covered for designated gastrointestinal organization.
A. Release Studies
Different prescriptions were coordinated into the mucoadhesive gadgets, and delivery tests were performed to determine their delivery profiles. The gadget ought to preferably deliver its items steadily and completely. Drug discharge profiles were estimated after different prescriptions were set onto gastrointestinal mucoadhesive gadgets included Eudragit® E PO, gelatin, and SCMC
B. Protein Release
In PBS with a pH of 7.4, the gadgets' delivery profiles for BSA and lysozyme were surveyed. These proteins were picked on the grounds that their charge states are particular at pH 7.4, their isoelectric point. The reason for the examination was to look at the two proteins' delivery profiles and make determinations about what the gadgets' charge settings mean for the proteins' capacity to be delivered. The investigation discovered that stacked proteins were quickly delivered inside the initial three hours, with a level prompting total protein discharge inside the following two hours
C. Insulin Release
The pace of FITC-insulin discharge in pH 7.4 PBS at 37°C was estimated after gadgets were stacked with the protein. In the main hour of the exploration, the gadgets delivered around 75% of the complete portion of the medication quick, trailed by a slow delivery over the course of the following 3 hours to arrive at 100 percent drug discharge by 4 hours
Conclusion
Insulin and an infiltration enhancer called PPS have been planned into an exceptional oral digestive mucoadhesive gadget. The gadgets showed fantastic mucoadhesive strength, enduring multiple times their own weight, and extensive blood glucose bringin down viability in vivo, and they had the option to deliver their whole medication load. Insulin can be taken orally with the assistance of digestive mucoadhesive gadgets for the treatment of diabetes, wiping out the requirement for everyday insulin infusions. The consequences of the review amount to the possibility that digestive mucoadhesive gadgets are a feasible option in contrast to insulin infusions for the administration of diabetes, which can impact the personal satisfaction for individual with diabetes .
References
[1] Marschütz MK, Bernkop?Schnürch A. Oral peptide drug delivery: polymer–inhibitor conjugates protecting insulin from enzymatic degradation in vitro .Biomaterials. 2000;21:1499–1507. doi:10.1016/S0142-9612(00)00039-9. [2] Banerjee A, Onyuksel H. Peptide delivery using phospholipid micelles. Wiley Interdiscip Rev NanomedNanobiotechnol. 2012;4:562–574. doi:10.1002/wnan.1185. [3] Carino GP, Mathiowitz E. Oral insulin delivery. Adv Drug Deliv Rev. 1999;35:249–257. doi:10.1016/S0169- 409X(98)00075-1. [4] Fu AZ, Qiu Y, Radican L. Impact of fear of insulin or fear of injection on treatment outcomes of patients with diabetes. Curr Med Res Opin. 2009;25:1413–1420. doi:10.1185/03007990902905724. [5] Gao X, Cao Y, Song X, et al. Biodegradable, pH?responsive carboxymethyl cellulose/poly(acrylic acid) hydrogels for oral insulin delivery. MacromolBiosci. 2014;14:565–575. doi:10.1002/mabi.201300384 [6] Goldberg M, Gomez?Orellana I. Challenges for the oral delivery of macromolecules. Nat Rev Drug Discov. 2003;2:289–295. doi: 10.1038/nrd1067. [7] Gupta, A., Singh, R., Sharma, V., & Verma, A. (2020). Development and characterization of a mucoadhesive patch for controlled insulin delivery using pectin from citrus fruit peel. International Journal of Pharmaceutics, 576, 118958. [8] Hamman JH, Enslin GM, Kotze AF. Oral delivery of peptide drugs: barriers and developments. BioDrugs. 2005;19:165– 177. [9] Hosny EA, Al?Shora HI, Elmazar MMA. Oral delivery of insulin from enteric?coated capsules containing sodium salicylate: effect on relative hypoglycemia of diabetic beagle dogs. Int J Pharm. 2002;237:71–76. doi:10.1016/S0378- 5173(02)00024-8 [10] Jain, A., Mishra, P., Jain, A., & Kumar, A. (2018). Design and evaluation of mucoadhesive patches for transbuccal delivery of metformin hydrochloride using gum karaya and gum tragacanth. Drug Development and Industrial Pharmacy, 44(6), 923-931. https://doi.org/10.1080/03639045.2018.1441563 [11] Khan, S., Gowda, D. V., & Ahmad, M. Z. (2021). Development and optimization of mucoadhesive patches of glibenclamide for buccal drug delivery: In vitro and in vivo evaluation. Saudi Pharmaceutical Journal, 29(2), 162-170. https://doi.org/10.1016/j.jsps.2020.12.008 [12] Krauland AH, Guggi D, Bernkop?Schnürch A. Oral insulin delivery: the potential of thiolated chitosan?insulin tablets on non?diabetic rats. J Control Release. 2004;95:547–555. doi:10.1016/j.jconrel.2003.12.017. [13] Morris AD, Boyle DI, McMahon AD, Greene SA, MacDonald TM, Newton RW. Adherence to insulin treatment, glycaemic control, and ketoacidosis in insulin?dependent diabetes mellitus. The DARTS/MEMO Collaboration. Diabetes Audit and ResearchinTayside Scotland. Medicines Monitoring Unit. Lancet. 1997;350:1505–1510. [14] Prabhu, P., & Mishra, D. K.(2019). Development and evaluation of mucoadhesive patches for transbuccal delivery of glimepiride using aloevera gel and karaya gum.Journal of drug delivery science and technology,54, 101273.
Copyright
Copyright © 2023 Tapade S. S, Priyanka P. Dappadwad, Priti M. Mane, Punam P. Suryawanshi, Rameshwar Sangle, Ramraje Lahade, Rutuja Tandle, Rutuja Bedre. This is an open access article distributed under the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.

Download Paper
Paper Id : IJRASET56312
Publish Date : 2023-10-26
ISSN : 2321-9653
Publisher Name : IJRASET
DOI Link : Click Here
 Submit Paper Online
Submit Paper Online

